Management of Side Effects
NULLAdverse effects: the other side of the coinWhen highly active antiretroviral treatment (HAART) become available in 1996, enthusiasm for this new treatment option for HIV-infection was paramount in all the developed world. Antiviral treatment was proposed to almost every patient given the profound effect of treatment on HIV-replication and survival. However, it became obvious from the very beginning, that success never remains alone. Already during the AIDS conference 1996 in Vancouver, investigators presented pictures of a clinical condition associated with indinavir (Crixivan®) treatment that was consequently termed “crix-belly”. Soon after the observation of this abdominal fat accumulation in patient under HAART, it became obvious that treatment with any antiviral combination did result in a profound disturbance of fat metabolism. Fat accumlation was often observed in parallel with symptoms of fat waisting (predominantly face and extremities) also known as lipoatrophy, therefore the term lipodystrophy was created for this combination of side effects in fat metabolism. Although the exact mechanisms of these disturbancies are still under investigation, it is clear that the observed pictures are not just a cosmetic problem but represent a clinical sign of a very profound disturbance of metabolic events that also influence several pathways of lipid and steroid metabolism and oxidative energy production. Dissecting lipoatrohpy from fat accumulationThe major breaktrough in our understanding of the complex basis of the observed side effect was made when the signs of lipoatrophy were separated from the other metabolic adverse events seen in the same patients. 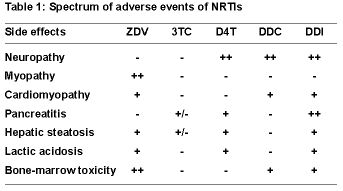 It was a young dutch scientist (Brinkmann) how first recognized that the clinical picture of lipoatrophy was just one aspect of a syndrome that resembled a genetic disorder of mitochondrial metabolism. Based on results from cohort studies but also from in-vitro experiments it became obvious that nukleotide analogue RT-inhibitors (NRTI) also inhibit the mitochondrial enzyme DNA polymerase and that this side effect may be responsible for a broad clinical spectrum of side effects, including, but not limited to lipoatrophy. For reasons that are not well known, inhibiting properties of NRTI on mitochondrial DNA differs in severity as well as by target organ. Or to cite Dr. David Wohl from Chapel Hill during the video-conference: Not all NRTIs are created equal. The spectrum of side effects of all NRTIs summarized in the table. The other spectrum of the lipodystrophy syndrome that needs to be dissected from lipoatrophy are fat accumulation and disturbances in the fat and insulin metabolism. Lipoatrophy: When the mirror tells you, you are sickThe first case that was carefully selected from the team in Basel (nicely presented by Christoph Strub) exemplifies the problems
It was a young dutch scientist (Brinkmann) how first recognized that the clinical picture of lipoatrophy was just one aspect of a syndrome that resembled a genetic disorder of mitochondrial metabolism. Based on results from cohort studies but also from in-vitro experiments it became obvious that nukleotide analogue RT-inhibitors (NRTI) also inhibit the mitochondrial enzyme DNA polymerase and that this side effect may be responsible for a broad clinical spectrum of side effects, including, but not limited to lipoatrophy. For reasons that are not well known, inhibiting properties of NRTI on mitochondrial DNA differs in severity as well as by target organ. Or to cite Dr. David Wohl from Chapel Hill during the video-conference: Not all NRTIs are created equal. The spectrum of side effects of all NRTIs summarized in the table. The other spectrum of the lipodystrophy syndrome that needs to be dissected from lipoatrophy are fat accumulation and disturbances in the fat and insulin metabolism. Lipoatrophy: When the mirror tells you, you are sickThe first case that was carefully selected from the team in Basel (nicely presented by Christoph Strub) exemplifies the problems 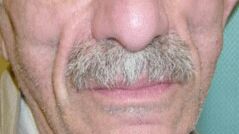 one is faced with a patient presenting with the prominent signs of facial atrophy due to long-time NRTI-treatment. So far, we have no indication, that this condition is more than a cosmetically disturbing one. However, patients obviously do not like the changes in their face (see the sunken cheeks in the figure at right). And when they realize it is the treatment that causes this change, they start to dislike their tablets, which in turn will jeopardize adherence and thus will limit the effect of HAART. Thus, patients need to be carefully informed about the nature of this conditions and options to improve it need to be developed. One option, also used in this case in Basel, is to change the composition of HAART. Since not all NRTIs are created equal, one might try to replace one NRTI with another one with a weaker tendency to produce lipodystrophy. The Carr study (see case report no.1) did investigate exactly this question and demonstrated a trend towards normalization in lipid measurements by DEXA-scans when one NRTI was replaced by abacavir. Most investigators would not consider stopping treatment as a valid option in a patient who had advanced disease before initiation of HAART. If ever possible, drug interruptions should only be recommended in the context of a clinical study. Therefore, if all options for changing NRTIs are used, most patients either have to live with this condition or have to get cosmetic correction through subcutaneous injections of polylacticacid or similar substances. However, as Dr. Flepp and Dr. Wohl pointed out in their discussions, patients and health care providers need to realize that the driving force behind all this is a deadly disease. Cardiovascular risk: a new concern in HIV infectionThe second case that was presented by Hans Hirsch from Basel pointed to another flip side of HAART. Over the past 8 years, this patient was treated with a number of antiretrovirals until he finally had a long-lasting treatment success (since 1999) with a combination of AZT, efavirenz and ritonavir-boosted indinavir (100/800). However, the last combination treatment resulted in an profound increase in cholesterol and triglyceride levels. This increase in blood lipids in the setting of an optimal antiviral treatment rises a second concern. Does the treatment induced elevation of lipids results in an increase of the cardiovascular risk, and if so, to what extent. The D.A.D. study, a multi-cohort approach to study cardiovascular side effects is currently addressing this issue (principal investigator for the SHCS: Rainer Weber, Zürich). However, one prominent finding in the baseline characteristics of over 20’000 study participants is the high prevalence of smokers (approx. 60%). In D.A.D the prevalence of hyperlipidemia is highest in patients treated with NRTI+NNRTI+PI. Given the lack of evidence addressing the additional risk of lipid alterations in HIV, the best advice is to treat hyperlipidemia in HIV positive patients exactly as in HIV-negatives (see case 2 for references). We thus assume that the 10-year risk for a cardiovascular event is 16% non-smokers with elevated cholesterol and up to 30% in smokers.
one is faced with a patient presenting with the prominent signs of facial atrophy due to long-time NRTI-treatment. So far, we have no indication, that this condition is more than a cosmetically disturbing one. However, patients obviously do not like the changes in their face (see the sunken cheeks in the figure at right). And when they realize it is the treatment that causes this change, they start to dislike their tablets, which in turn will jeopardize adherence and thus will limit the effect of HAART. Thus, patients need to be carefully informed about the nature of this conditions and options to improve it need to be developed. One option, also used in this case in Basel, is to change the composition of HAART. Since not all NRTIs are created equal, one might try to replace one NRTI with another one with a weaker tendency to produce lipodystrophy. The Carr study (see case report no.1) did investigate exactly this question and demonstrated a trend towards normalization in lipid measurements by DEXA-scans when one NRTI was replaced by abacavir. Most investigators would not consider stopping treatment as a valid option in a patient who had advanced disease before initiation of HAART. If ever possible, drug interruptions should only be recommended in the context of a clinical study. Therefore, if all options for changing NRTIs are used, most patients either have to live with this condition or have to get cosmetic correction through subcutaneous injections of polylacticacid or similar substances. However, as Dr. Flepp and Dr. Wohl pointed out in their discussions, patients and health care providers need to realize that the driving force behind all this is a deadly disease. Cardiovascular risk: a new concern in HIV infectionThe second case that was presented by Hans Hirsch from Basel pointed to another flip side of HAART. Over the past 8 years, this patient was treated with a number of antiretrovirals until he finally had a long-lasting treatment success (since 1999) with a combination of AZT, efavirenz and ritonavir-boosted indinavir (100/800). However, the last combination treatment resulted in an profound increase in cholesterol and triglyceride levels. This increase in blood lipids in the setting of an optimal antiviral treatment rises a second concern. Does the treatment induced elevation of lipids results in an increase of the cardiovascular risk, and if so, to what extent. The D.A.D. study, a multi-cohort approach to study cardiovascular side effects is currently addressing this issue (principal investigator for the SHCS: Rainer Weber, Zürich). However, one prominent finding in the baseline characteristics of over 20’000 study participants is the high prevalence of smokers (approx. 60%). In D.A.D the prevalence of hyperlipidemia is highest in patients treated with NRTI+NNRTI+PI. Given the lack of evidence addressing the additional risk of lipid alterations in HIV, the best advice is to treat hyperlipidemia in HIV positive patients exactly as in HIV-negatives (see case 2 for references). We thus assume that the 10-year risk for a cardiovascular event is 16% non-smokers with elevated cholesterol and up to 30% in smokers.  Hyperlipidemia is just one aspect of the broad spectrum of adverse events observed under HAART. It is currently still not known, whether fat accumulation (usually truncal obesity, “crix-belly”, buffolo hump {see picture}, breast enlargement) and lipid abnormalities share a common pathway. Protease inhibitors have been shown to inhibit enzymes in the sterol-binding pathway which may also explain in part hyperglycemia that is also observed under protease inhibitor therapy. However, since NNRTI have a clear additive effect on hyperlipidemia, other not yet recognized mechanisms might also play an important role. Similar to lipodystrophy, there are only few other management options for a patient with hyperlipidemia. The three options are: 1) continue HAART and treat side effects symptomatically, 2) stop HAARt or 3) modify HAART These options need to be discussed with the patient. Other treatment options, however, are limited. The new protease inhibitor atazanavir looks promising in terms of lipid profile changes, but it remains to be shown whether this drug competes well with the currently available standards in terms of drug potency. Primum nihil nocereFirst rule in medical treatment is to avoid harm. The multiple adverse events observed with HAART seem to be a contradiction to this credo. However, harm induced by HAART needs to be counterbalanced with the benefits of treatment. Thus, decisions on treatment initiation and treatment changes must always consider drug interactions, side-effects and the risk of disease progression. PD Dr. med. Pietro Vernazza
Hyperlipidemia is just one aspect of the broad spectrum of adverse events observed under HAART. It is currently still not known, whether fat accumulation (usually truncal obesity, “crix-belly”, buffolo hump {see picture}, breast enlargement) and lipid abnormalities share a common pathway. Protease inhibitors have been shown to inhibit enzymes in the sterol-binding pathway which may also explain in part hyperglycemia that is also observed under protease inhibitor therapy. However, since NNRTI have a clear additive effect on hyperlipidemia, other not yet recognized mechanisms might also play an important role. Similar to lipodystrophy, there are only few other management options for a patient with hyperlipidemia. The three options are: 1) continue HAART and treat side effects symptomatically, 2) stop HAARt or 3) modify HAART These options need to be discussed with the patient. Other treatment options, however, are limited. The new protease inhibitor atazanavir looks promising in terms of lipid profile changes, but it remains to be shown whether this drug competes well with the currently available standards in terms of drug potency. Primum nihil nocereFirst rule in medical treatment is to avoid harm. The multiple adverse events observed with HAART seem to be a contradiction to this credo. However, harm induced by HAART needs to be counterbalanced with the benefits of treatment. Thus, decisions on treatment initiation and treatment changes must always consider drug interactions, side-effects and the risk of disease progression. PD Dr. med. Pietro Vernazza